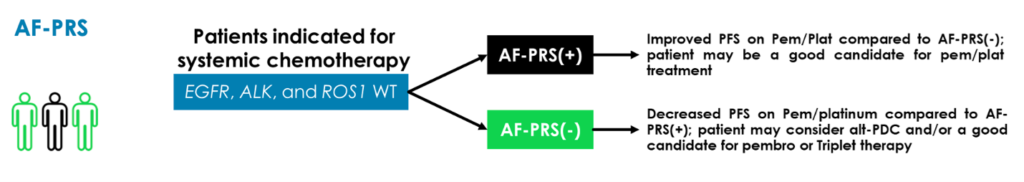
The Antifolate Predictive Response RNA Gene Signature (AF-PRS) is under development to address a clinical need in non-small cell lung cancer (NSCLC) treatment. Pemetrexed/platinum doublet chemotherapy (PMX-PDC) is a well-tolerated and effective chemotherapy for the treatment of non-squamous NSCLC. While it has a better adverse events profile than triplet therapy, there is currently no test to identify the patients most likely to respond.
AF-PRS is 48-gene RNA expression assay for FFPE tumor samples that identifies lung adenocarcinoma tumor subtypes associated with prognosis on PMX-PDC. It is intended as an adjunct to conventional clinical variables and models for determining prognosis of patients indicated for systemic chemotherapy.
There are several publications demonstrating the target biology of AF-PRS, including PMID: 25250715, 23722170, 37233991 and a Phase 2 study in patients with non-squamous NSCLC (IIIB/IV) treated with pemetrexed and cisplatin. The clinical study showed increased survival on PMX-PDC associated with the AF-PRS(+) subtype (PMID: 25250715).
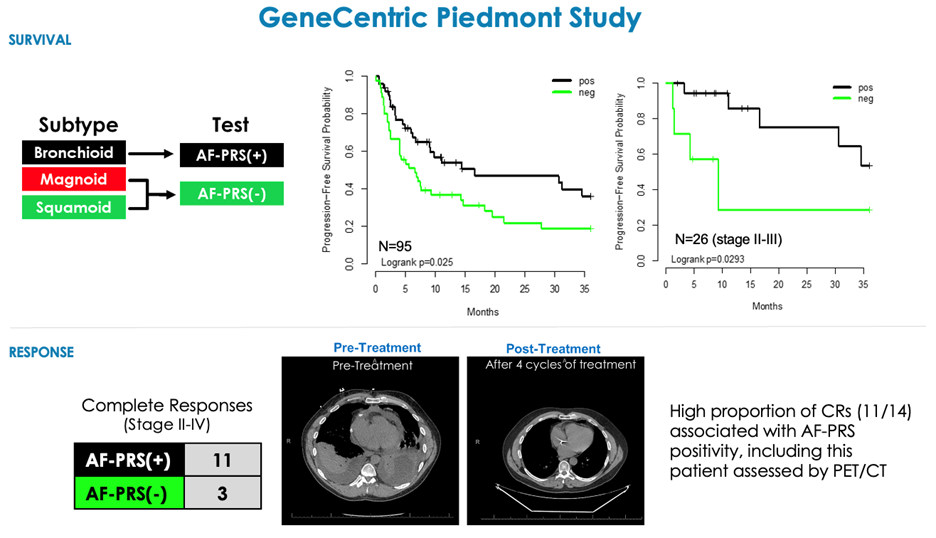
GeneCentric also recently completed the Piedmont study, a retrospective real-world evidence study in patients with non-squamous NSCLC (II-IV) treated with PMX-PDC. The study demonstrated increased survival and response to PMX-PDC treatment in the AF-PRS(+) subtype compared to the AF-PRS(-) subtype (Eisner et al 2023, Clinical Cancer Research, PMID: 37233991).