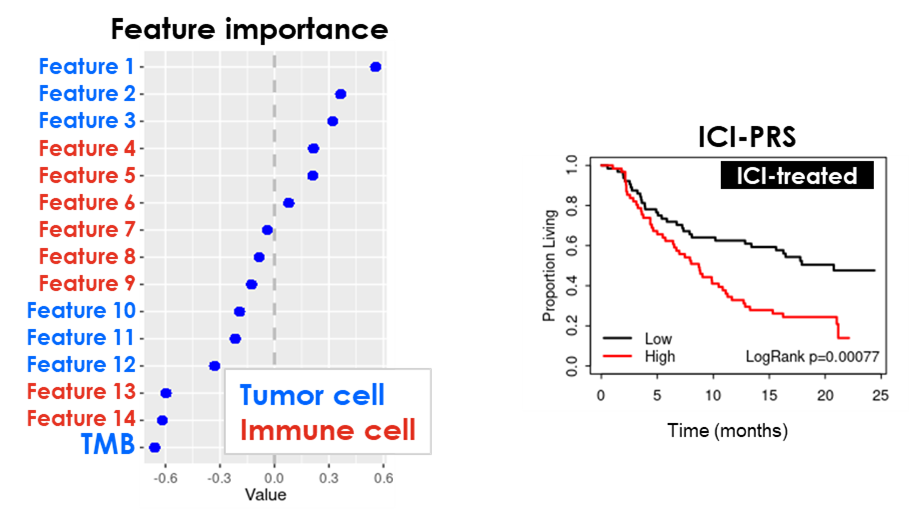
In many solid tumors, a relatively small proportion of eligible patients show durable responses to immune checkpoint inhibitor (ICI) therapy, and a lack of true predictive biomarkers for patient selection has led to several failed confirmatory survival studies. Biomarkers, such as TMB and PD-L1 positivity, point to responsive patients but are accompanied by high false positive and negative rates.
GeneCentric has developed an Immune Checkpoint Inhibitor Predictive Response Signature (ICI-PRS) with genes associated with improved survival in ICI-treated patients, but not in standard-of-care treated patients. This predictive multi-feature signature is comprised of immune and tumor biology that can complement TMB and is vastly superior to current PD-L1 biomarker tests. The performance has been replicated in multiple external data sets. ICI-PRS is currently in development with a diagnostic partner.