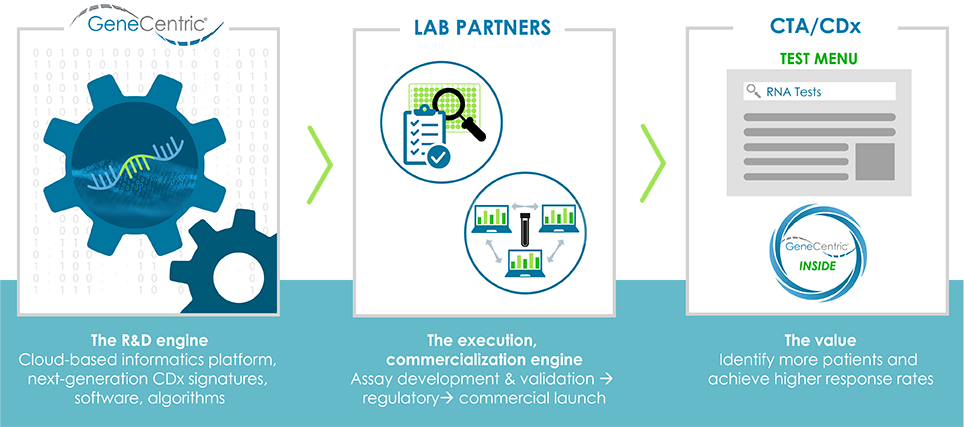
Biopharma Partnerships
We bring our expertise in discovery and development of RNA-based gene signatures to highly customized partnerships with biopharma companies. Partnerships can span discovery of de novo signatures for new targets and programs, application of an existing signature to an ongoing program, or managing the development of a signature into a CTA or CDx. Our partners range from early-stage biotechs to the largest pharmaceutical companies, where we leverage our vast experience to deliver program-enabling RNA-based gene signatures and diagnostics.