Our Science
Better biomarkers are needed to realize the promise of precision oncology
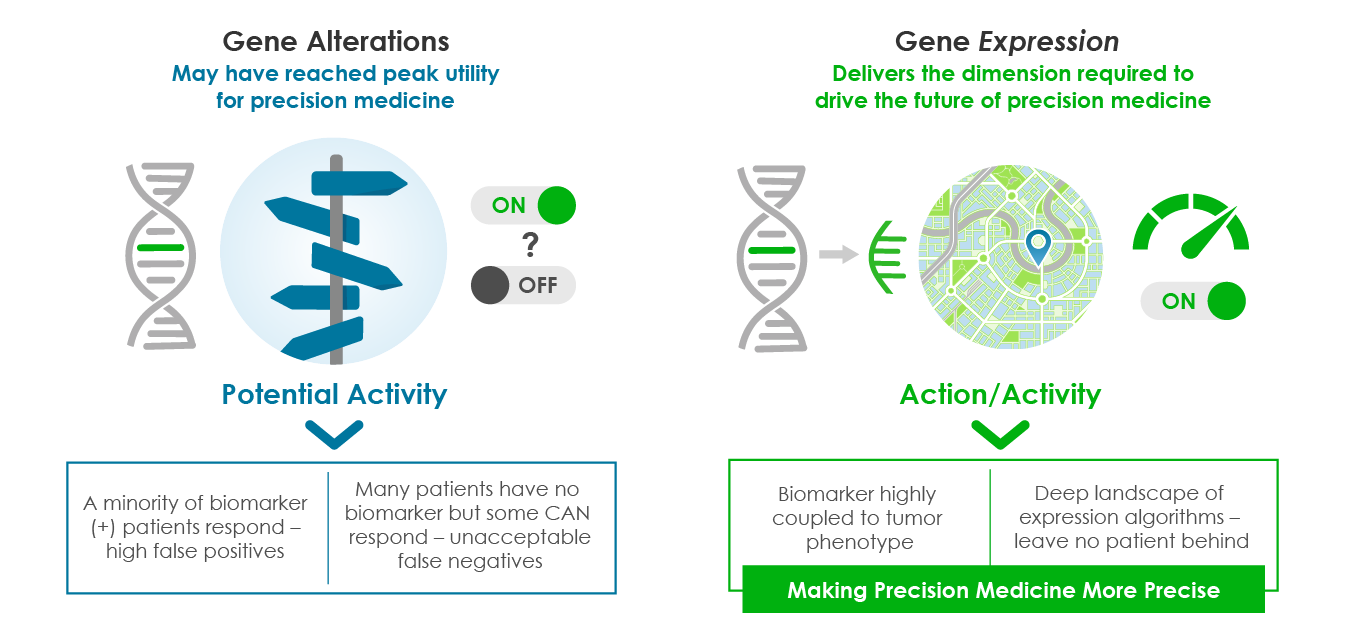
DNA alterations and other traditional biomarkers such as immunohistochemistry (IHC) may be the best option today but fall short of truly delivering precision medicine for most patients. Although these biomarkers invariably enrich for response, most biomarker-positive patients fail to respond to therapies, while significant numbers of biomarker-negative patients do. We believe the next era of precision oncology must go deeper.

Liquid biopsy is a fast-growing, multi-billion-dollar market, but current assays miss critical biology hidden in gene expression. Most tests rely solely on static DNA alterations. We’ve added dynamic gene expression – turning passive genomic data into active, intelligent data to overcome current limitations in liquid biopsies for precision oncology.
Gene expression: illuminating the tumor’s “on” state
Other functional drivers of response appear buried deep within tumor biology “dark matter.” Although DNA alterations as biomarkers will continue to be important, there is a need for biomarkers that more dynamically capture tumor complexity. Ground-shifting innovations are needed to help identify the precise biological characteristics of a patient’s tumor for accurate prognosis and therapy selection.
Unlike static DNA mutations, gene expression reveals the dynamic biological activity of a tumor’s “on” state to deliver deeper insights and more precise biomarkers. In practice, having both DNA alterations AND gene expression provides a clearer picture than either one alone.
Bringing gene expression to liquid biopsy
Genomic testing of tissue has become integral to contemporary oncology. As adoption grows, so does liquid biopsy. As a minimally invasive method for tumor profiling, it has become a critical adjunct to tissue testing – particularly in cases where tissue is limited or inaccessible – for defining the care plan and for clinical trials.
However, most liquid biopsy assays have the same liability as tissue genomic tests – they rely almost exclusively on detecting DNA alterations.
While RNA sequencing and gene expression augment tissue-based assays (e.g., fusion detection), the availability of gene expression data lags for liquid biopsies. This limitation restricts liquid biopsy assays to fully inform treatment decisions and highlights a significant opportunity to expand their capabilities to include comprehensive gene expression profiling.
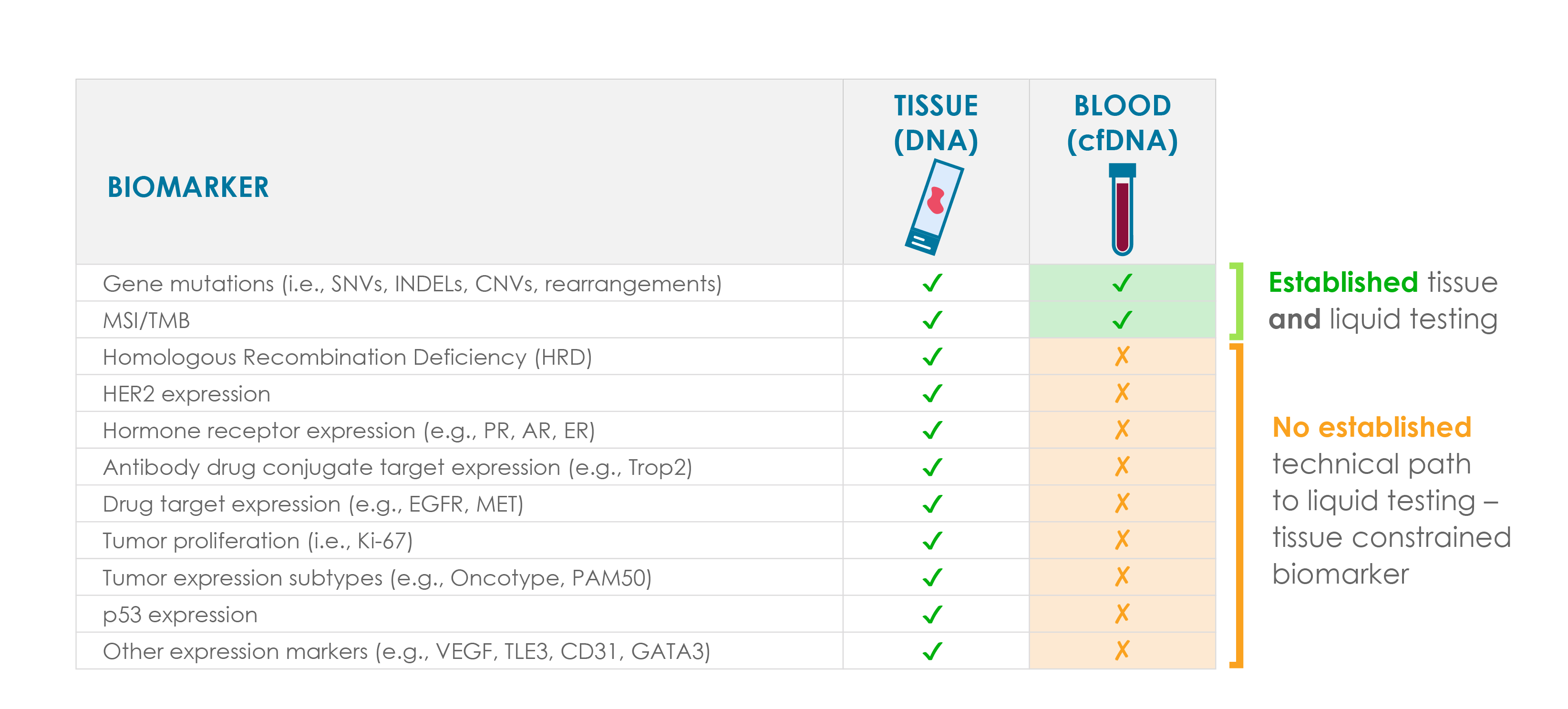
Our technology offers a solution, bringing gene expression to liquid biopsy in a simple, powerful way.
ExpressCT™ technology and the GenomicsNext™ platform bring gene expression to liquid biopsy
The union of pioneering innovations, proprietary pipelines, AI and machine learning comprises our ExpressCT technology and GenomicsNext platform, an integrated ctDNA platform for delivering conventional gene alteration data along with an entire second dimension of genomic data – gene expression. By adding gene expression data to ctDNA analysis, researchers and clinicians can derive more insights and value from a single sample.
We built ExpressCT using a deep library of proprietary tissue RNA signatures – giving us unique benchmarking power to engineer, train, and validate our technology. Without this foundation, others face years of data generation and guesswork just to begin.
The GenomicsNext platform aims to fuel new patient, biomarker and drug development insights. Its multimodal clinical-genomic data will be housed in a proprietary database with tools to enable real-world data access, exploration, and discovery.
Learn MoreGene expression products empower precision oncology
For over a decade, GeneCentric has developed technology, intellectual property and expertise in gene expression with a product pipeline that includes PurIST®, a commercialized tissue profiling test to inform pancreatic ductal adenocarcinoma standard of care, launched with Tempus. Our current focus brings our technology and expertise to ctDNA and liquid biopsy.
Learn More